FILSPARI® (sparsentan)
PROTECT (Phase 3 Study): Study Design & Results
PROTECT overview
PROTECT is a large, international, randomized, double-blind, active-controlled Phase 3 trial 1
Objective: To evaluate the long-term efficacy and safety of sparsentan in slowing kidney function decline in patients with IgA nephropathy who are at high risk of progression due to proteinuria ≥1 g/day despite treatment with RASi 1

Clinical significance:
Sparsentan could be used as a long-term foundational therapy for IgA nephropathy to reduce proteinuria and preserve kidney function 1

Key findings:
Compared to maximum labeled dose irbesartan (n=202), sparsentan (n=202) demonstrated 1* :

Maintenance of rapid and superior reduction of proteinuria 1

Better preservation of kidney function that accrued year on year 1, 2
Better projected eGFR data suggesting prolonged kidney survival 1, 2

A comparable safety profile 1
Footnotes:
*The target doses (sparsentan 400 mg and irbesartan 300 mg) were reached by 192 (95%) patients in the sparsentan group and 196 (97%) patients in the irbesartan group. 1
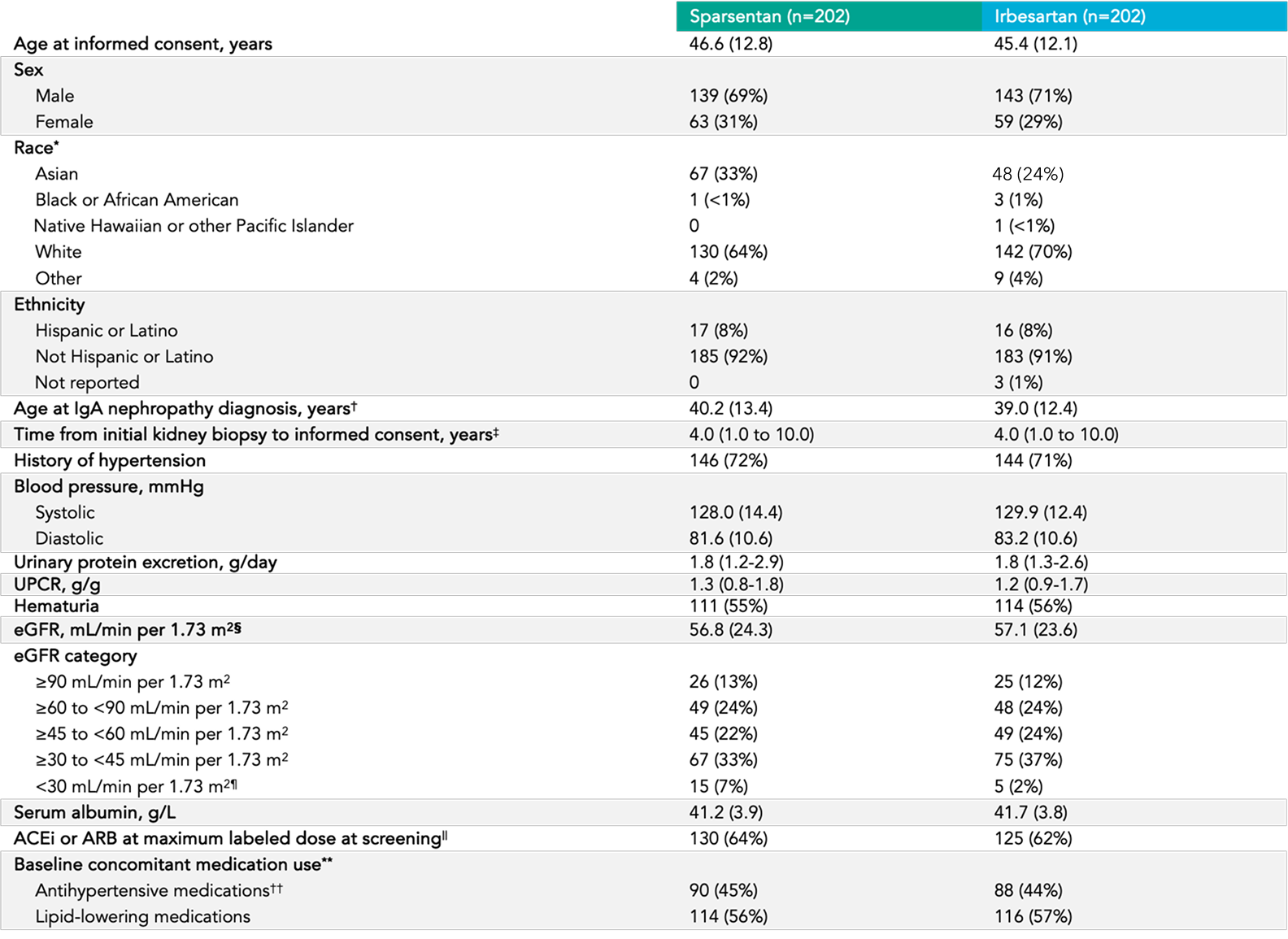
Baseline characteristics
View tableWell-balanced demographic, clinical and biochemical characteristics, and concomitant medication status 6
Mean age 5, 6
46 years
(range 18-76)
Sex 5
70% male
30% female
Race 5
67% white
28% Asian
1% Black or African American

Mean eGFR 5, 6
57 mL/min/1.73 m2

Proteinuria 5
1.2 g/g mean UPCR
49 (12%) patients
had UPCR > 3.5 g/24 hours

History of hypertension 5
~78% of patients

History of diabetes or IFG 5
11% of patients

Hematuria 5
56% of patients
Efficacy
Interactive Figures
Please select and start exploring
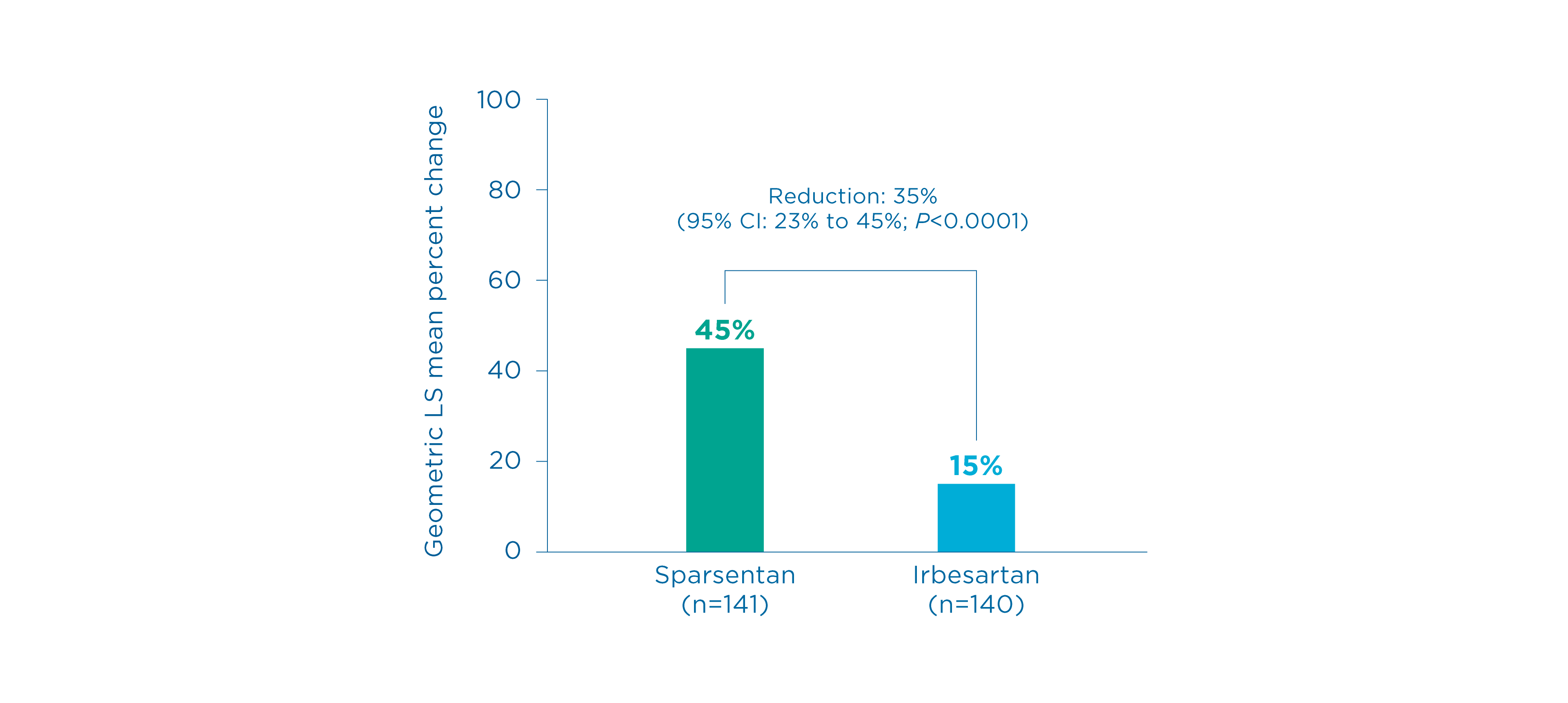
Proteinuria (UPCR) at Week 36
Primary endpoint
Change in UPCR from baseline up to Week 36 5, 7
Summary
- There was a mean reduction of proteinuria of 45% from baseline vs.15% in those receiving irbesartan 5
- The mean percent change in UPCR from baseline by sparsentan was significantly greater vs. irbesartan, corresponding to a 35% reduction (95% CI: 23% to 45%, P<0.0001) 5
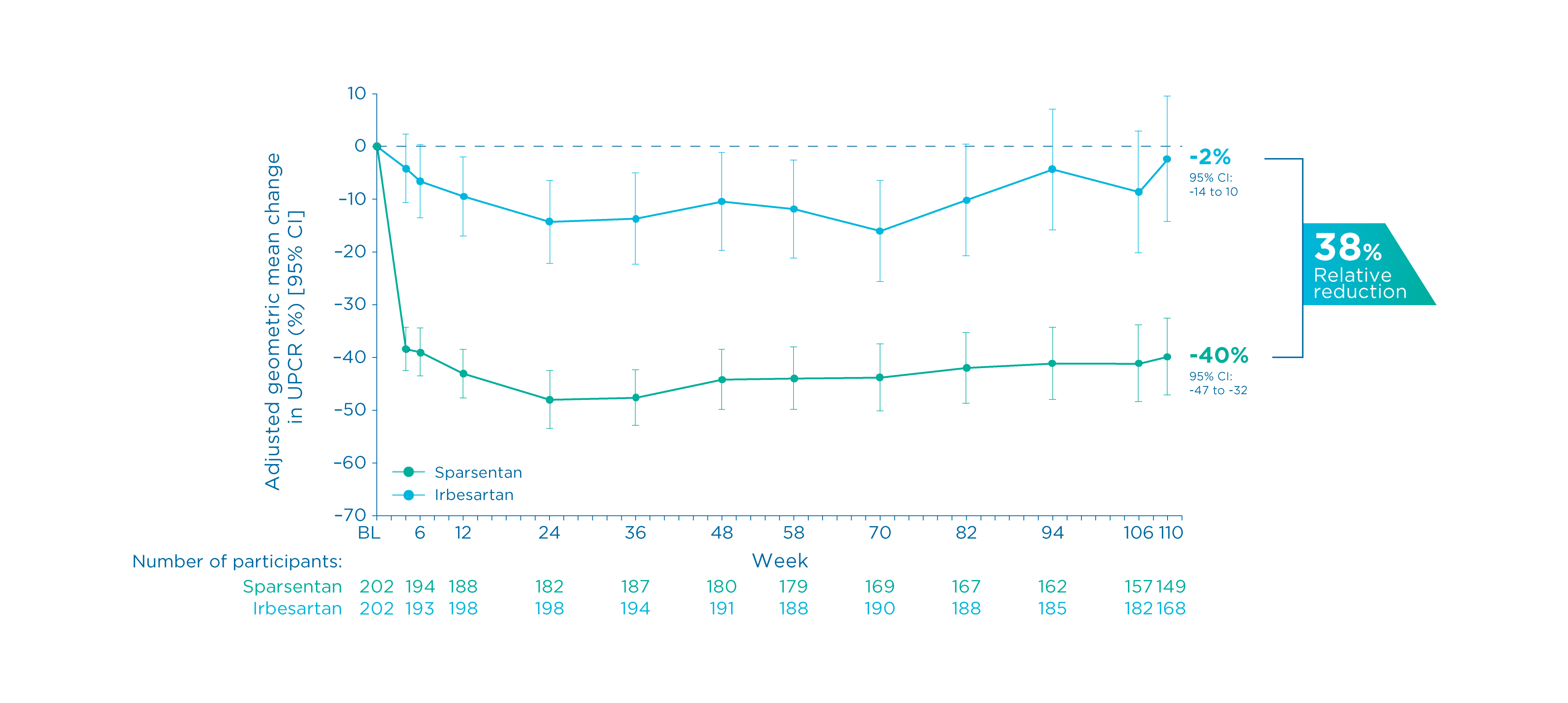
Proteinuria (UPCR) at Week 110
Other prespecified secondary efficacy endpoint
Changes in UPCR from baseline up to Week 110 3, 5
Summary
- There was a mean reduction of proteinuria of 40% from baseline in patients receiving sparsentan vs. 2% in those receiving irbesartan 3, 5
- The mean percent change in UPCR from baseline by sparsentan was significantly greater vs. irbesartan, corresponding to a 38% relative reduction
3
- The geometric LS mean ratio was 0.62 (95% CI: 0.52 to 0.73, P<0.0001) 3
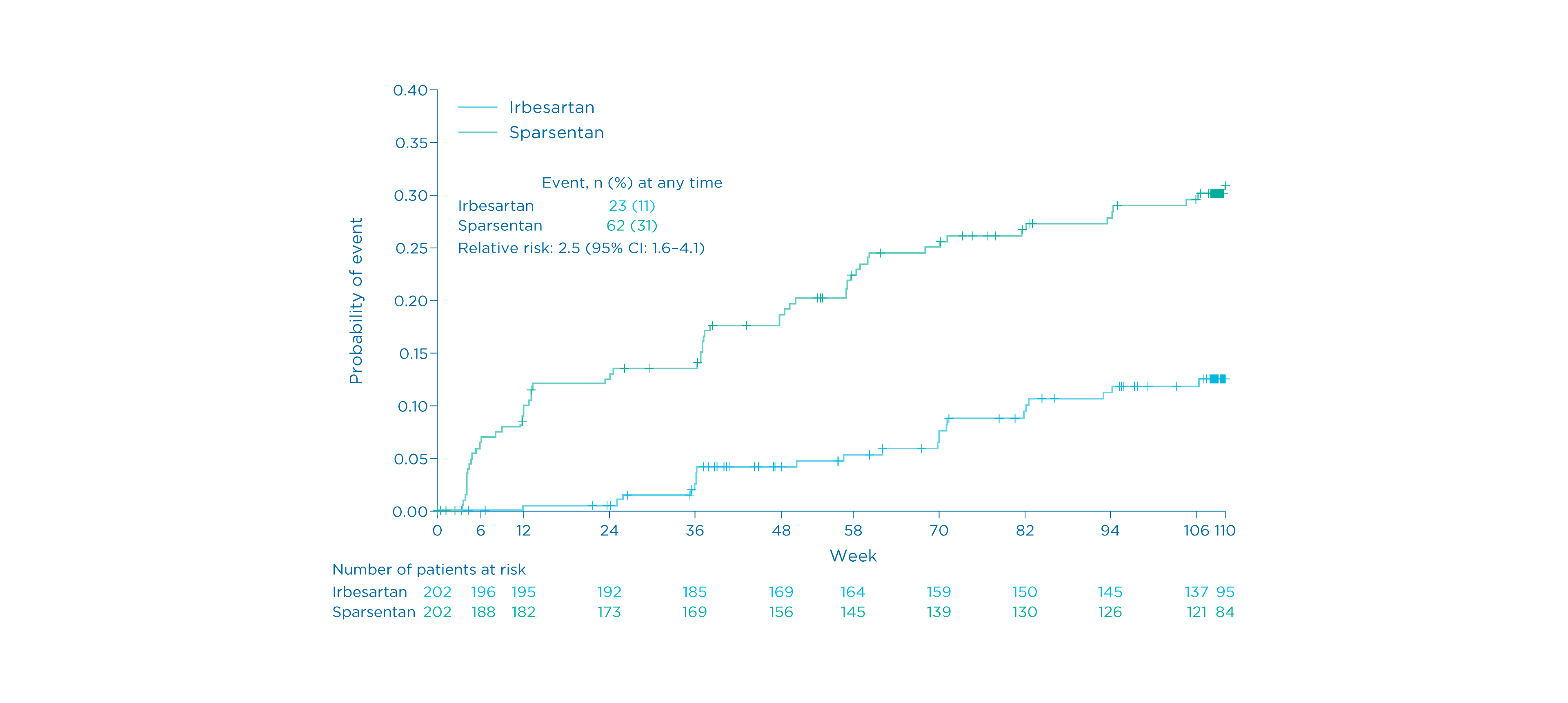
Time to complete remission of proteinuria at Week 110
Prespecified exploratory endpoint
Time to achieve complete proteinuria remission 2
Summary
- Sixty-two (31%) patients receiving sparsentan achieved complete remission (UPE <0.3 g/day) vs. 23 (11%) of those receiving irbesartan 1
- Patients in the sparsentan group achieved complete remission earlier and more frequently than those in the irbesartan group 1
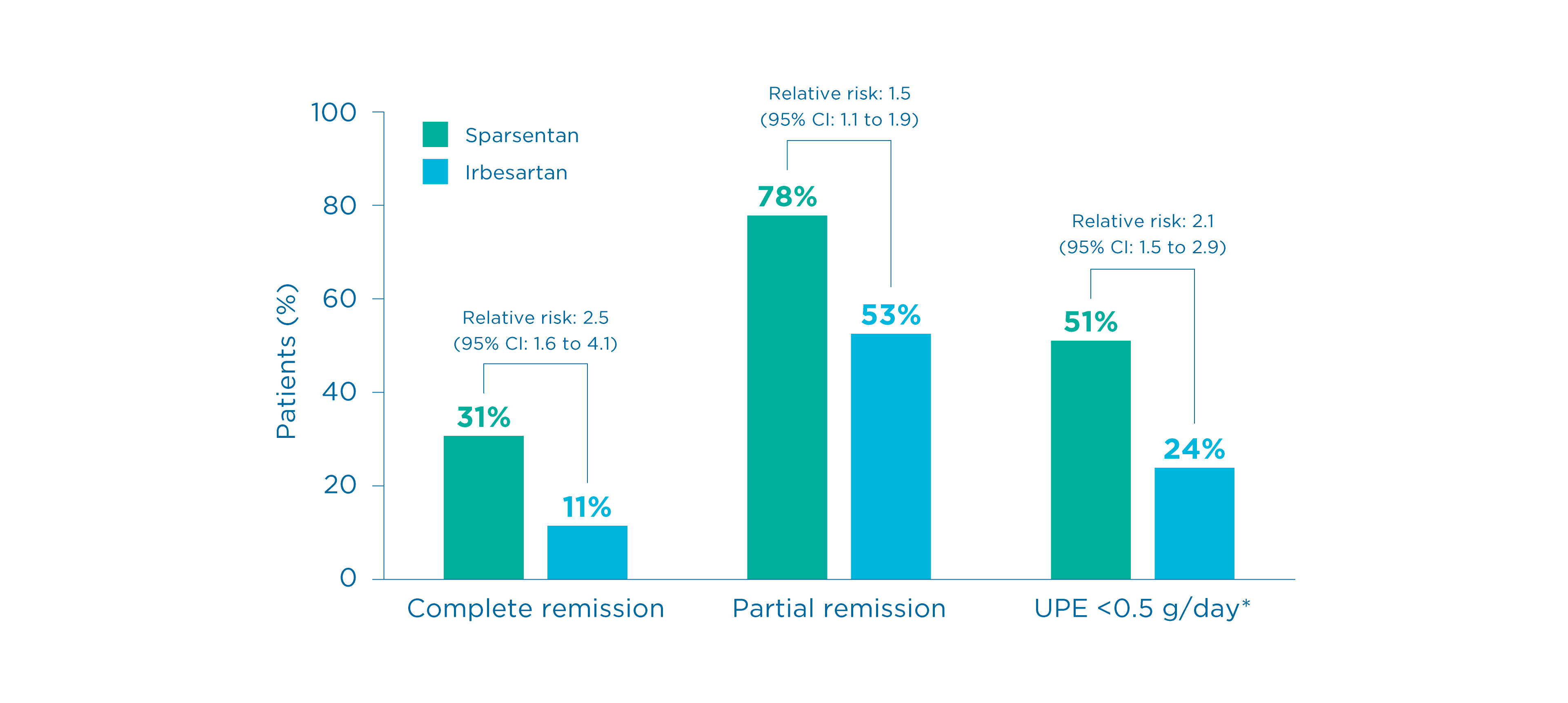
Complete and partial remission of proteinuria at Week 110
Prespecified exploratory endpoint
Complete and partial proteinuria remission 2
Summary
- More patients in the sparsentan group achieved complete remission (UPE <0.3 g/day) and partial proteinuria (UPE <1.0 g/day) compared to the irbesartan group 1
- Findings for partial remission were consistent with observations for complete remission 8
Footnotes
*The proportion of patients achieving UPE <0.5 g/day was a post hoc assessment 2
Estimated eGFR chronic slope or total slope at Week 110
Prespecified key secondary efficacy endpoint
Chronic slope 9
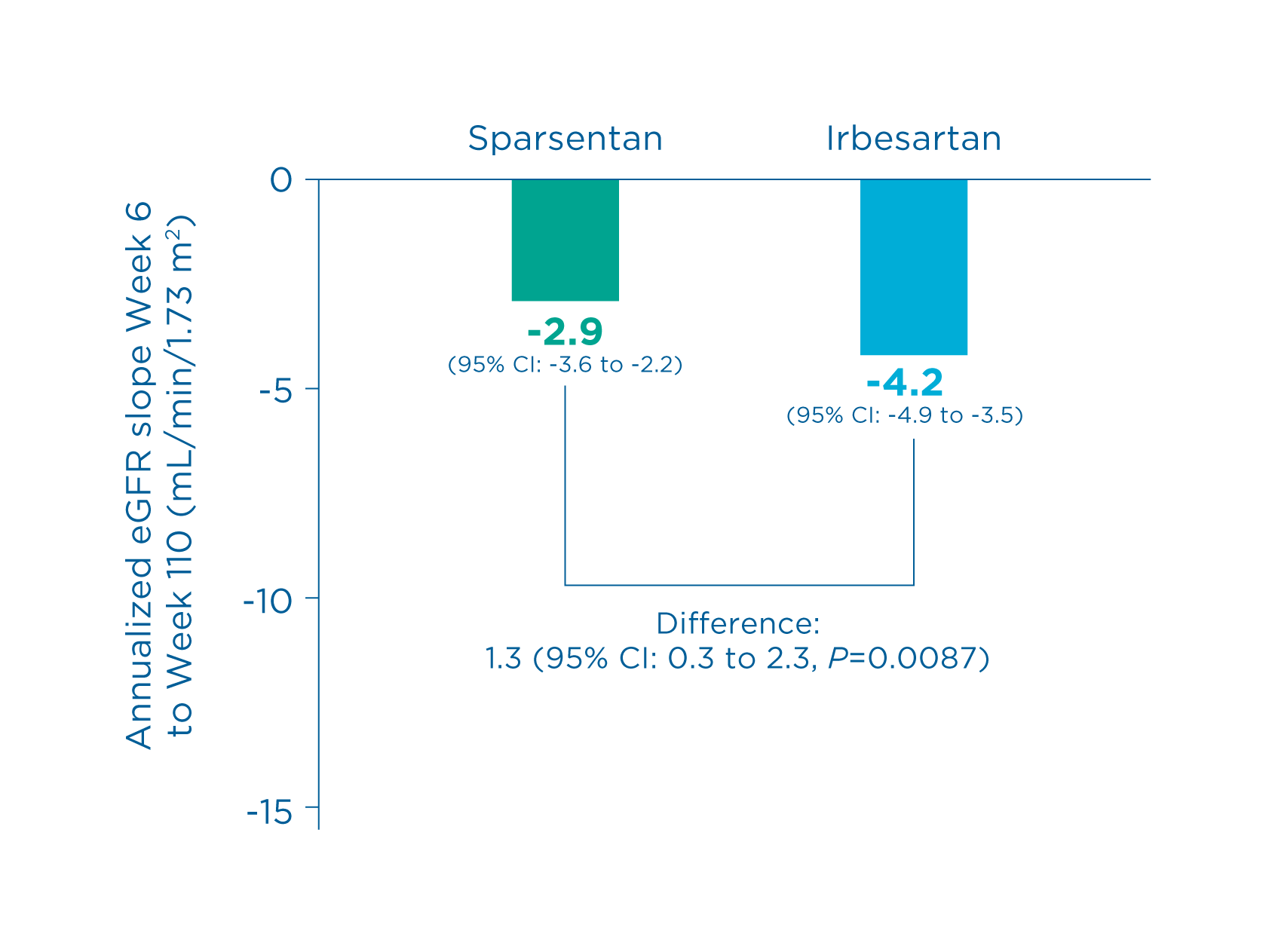
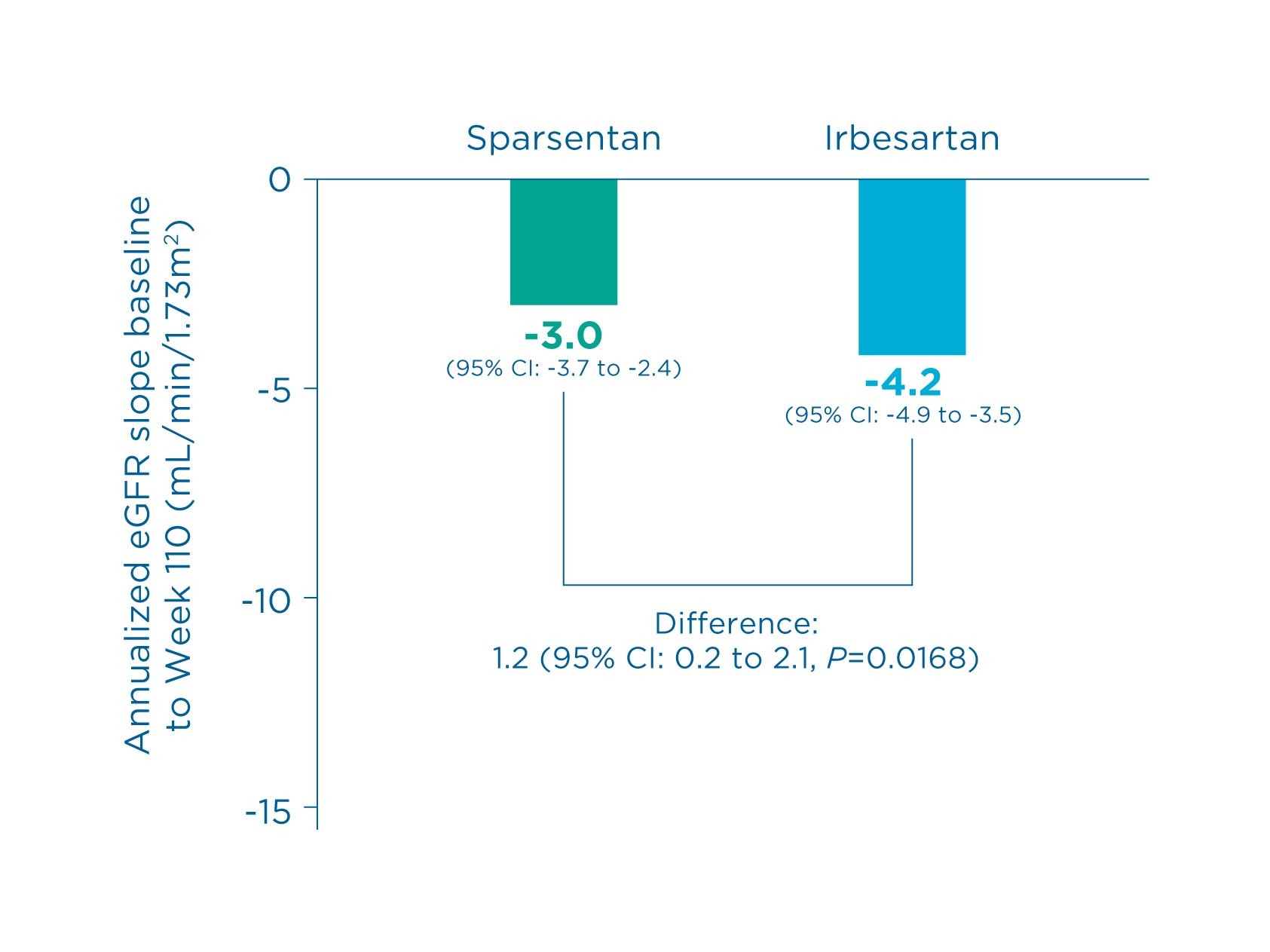
Total slope 9
Summary
- Sparsentan showed a statistically significant reduction vs irbesartan in the rate of eGFR decline
5,
9
- Total slope (sparsentan vs. irbesartan): -3.0 vs. -4.2 (Difference: 1.2, 95% CI: 0.2 to 2.1, P=0.0168) 9
- Chronic slope (sparsentan vs. irbesartan): 2.9 vs. -4.2 (Difference: 1.3, 95% CI: 0.3 to 2.3, P=0.0087) 9
Kidney function (eGFR) at Week 110
Other prespecified secondary efficacy endpoint
Change in eGFR by visit up to Week 110 4, 5*†
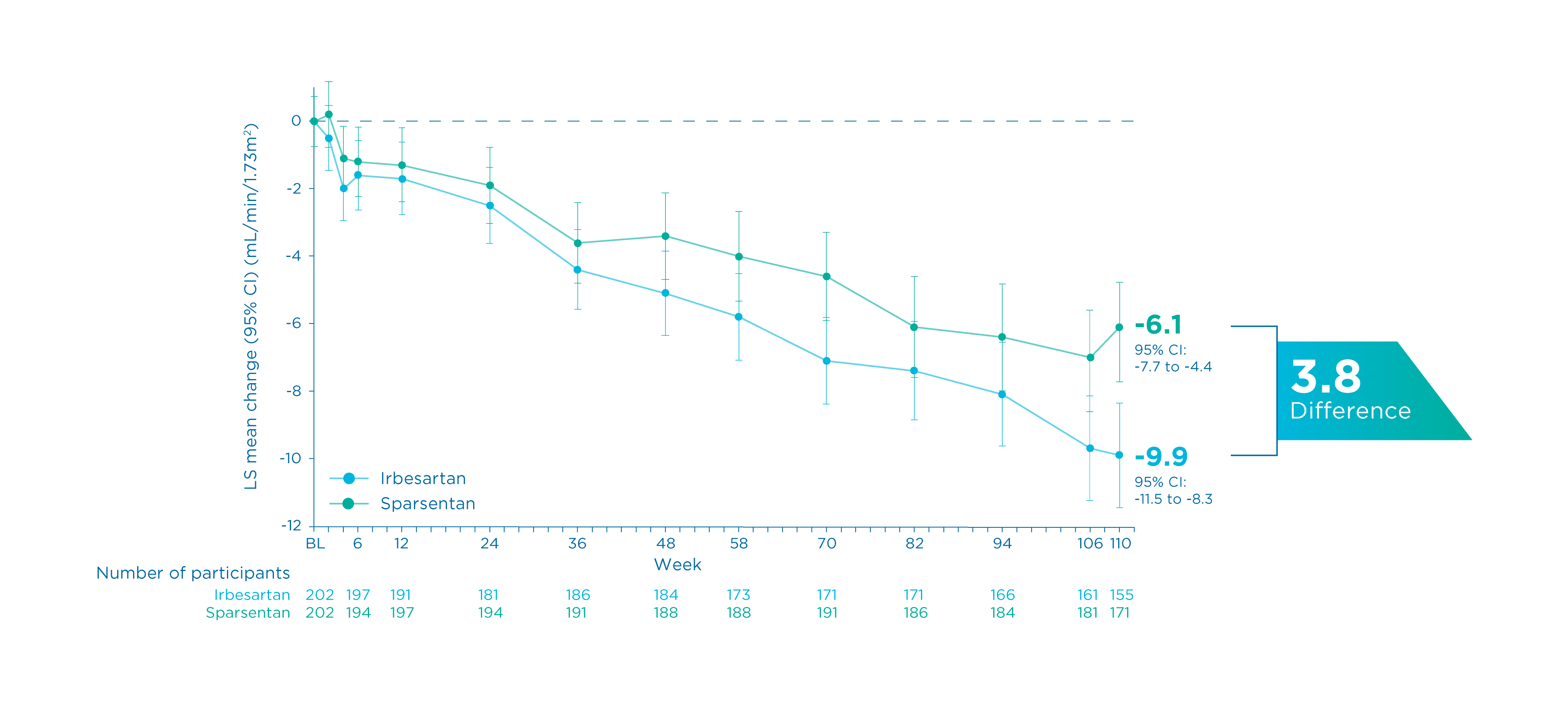
Summary
- At Week 110, there was a lower absolute change from baseline in eGFR with sparsentan vs. irbesartan 4, 5
- Sparsentan vs. irbesartan: -6.1 vs. -9.9 (Between-group difference: -3.8, 95% CI: 1.6 to 6.1) 4
Footnotes
*eGFR was calculated using the CKD-EPI equation. Baseline was defined as the last non-missing observation on or prior to the start of dosing. The analysis includes eGFR data during the double-blind period up to Week 110 regardless of treatment discontinuation or immunosuppressive therapy initiation. 5
†The treatment benefit with sparsentan on the rate of change in eGFR through Week 110 was not evident in patients with an eGFR ≥90 mL/min/1.73 m2; however, there was a small number of patients in this subgroup. 5
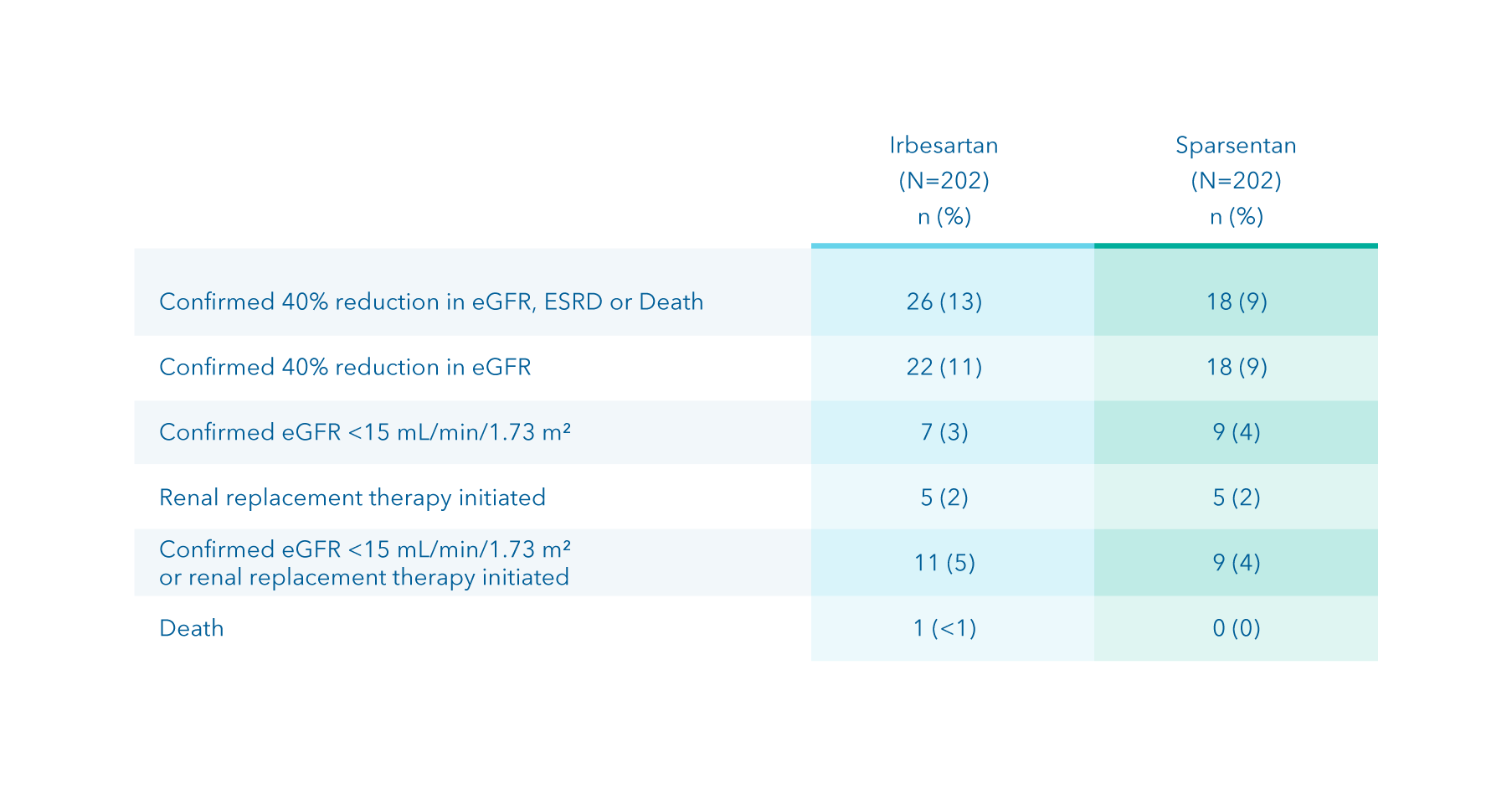
Composite kidney failure endpoint at Week 110
Other prespecified secondary efficacy endpoint
Time to reach the composite kidney failure endpoint 1*
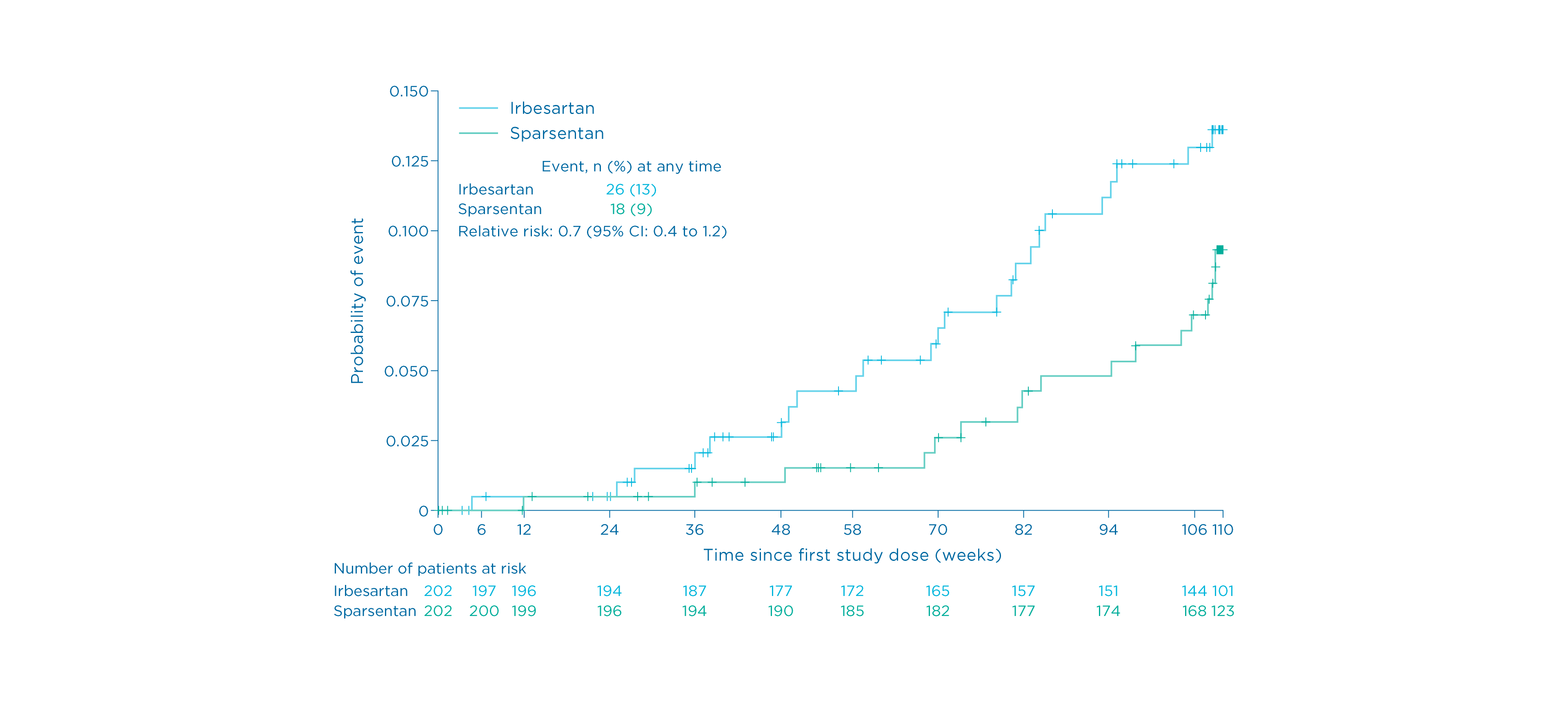
Summary
- Eighteen (9%) patients receiving sparsentan reached the composite kidney failure endpoint vs. 26 (13%) of those receiving irbesartan 1
Footnotes
*Defined as confirmed 40% reduction in eGFR, end-stage kidney disease, or death.
1
Potential long-term impact of improved eGFR slope
Additional analysis
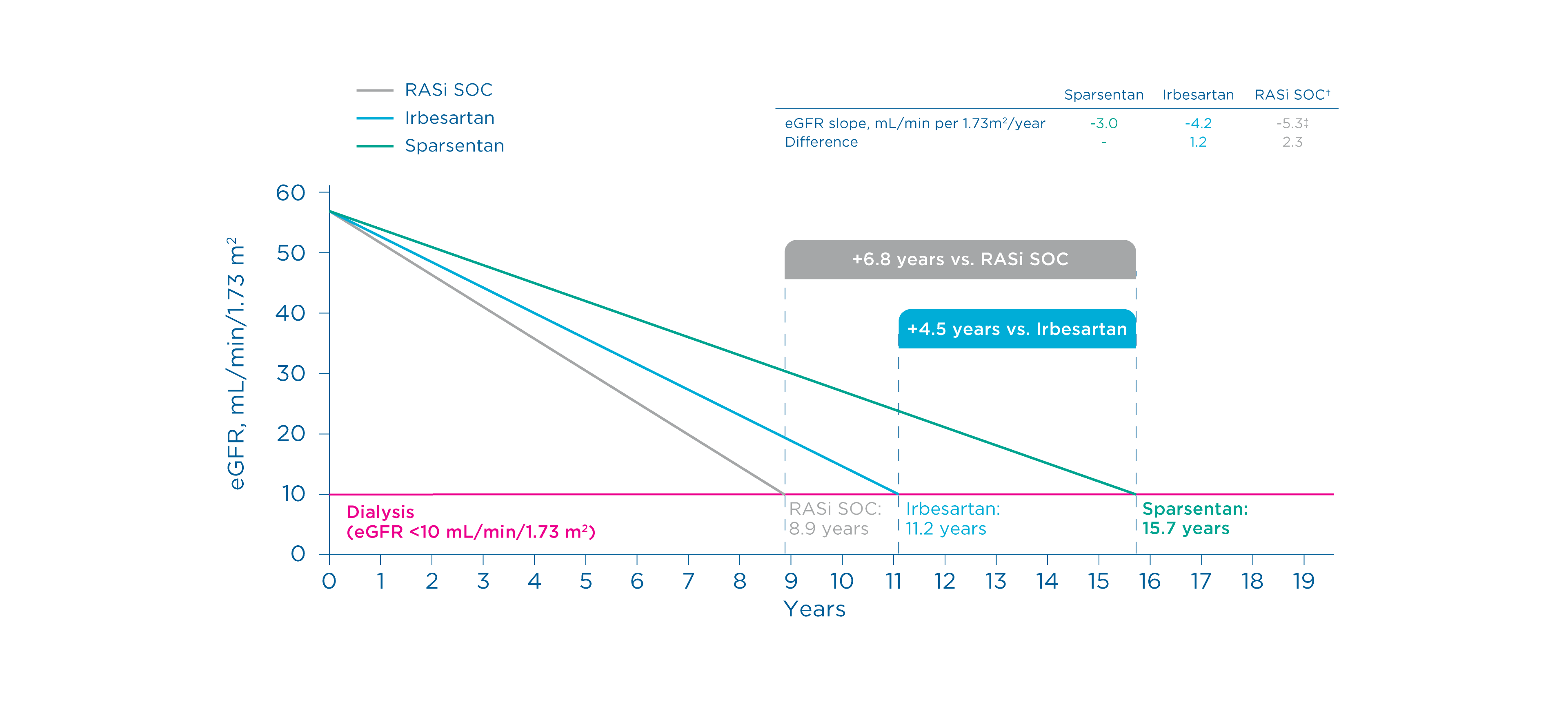
Potential long-term impact of improved eGFR slope 2, 5*
Summary
Projected eGFR slopes for sparsentan and irbesartan were derived from the PROTECT study 1, 2
Footnotes
*Baseline eGFR was set to equal 57 mL/min/1.73 m2 (0 years), reflecting the mean eGFR of all patients (N=404) reported in this study. 2
†ACEi and/or ARB. 2
‡Mean of observed chronic or total slopes for SOC ACEi/ARB as reported in 5 randomized controlled trials in IgA nephropathy. 2
§DAPA-CKD was a multicenter, double-blind, placebo-controlled, randomized trial designed to assess the effects of dapagliflozin on kidney and cardiovascular outcomes in patients with CKD, with or without type 2 diabetes. Data in this graph reflects patients with IgA nephropathy. 10
¶This study was a prospective, open-label, randomized-controlled trial designed to assess the effect of adding a 6-month course of corticosteroids to long-term ramipril use on renal survival and function. 11
#TESTING was a multicenter, double-blind, randomized-controlled trial designed to assess the effect of oral methylprednisolone on kidney function in patients with IgA nephropathy. 12
**HKVIN was a multicenter, double-blind, placebo-controlled, randomized trial designed to assess the therapeutic role of valsartan in patients with IgA nephropathy. 13
††NefIgArd was multicenter, double-blind, placebo-controlled, randomized trial designed to assess the effect of Nefecon on kidney function in patients with IgA nephropathy. 14
Use of systemic IST through Week 110
Prespecified exploratory endpoint
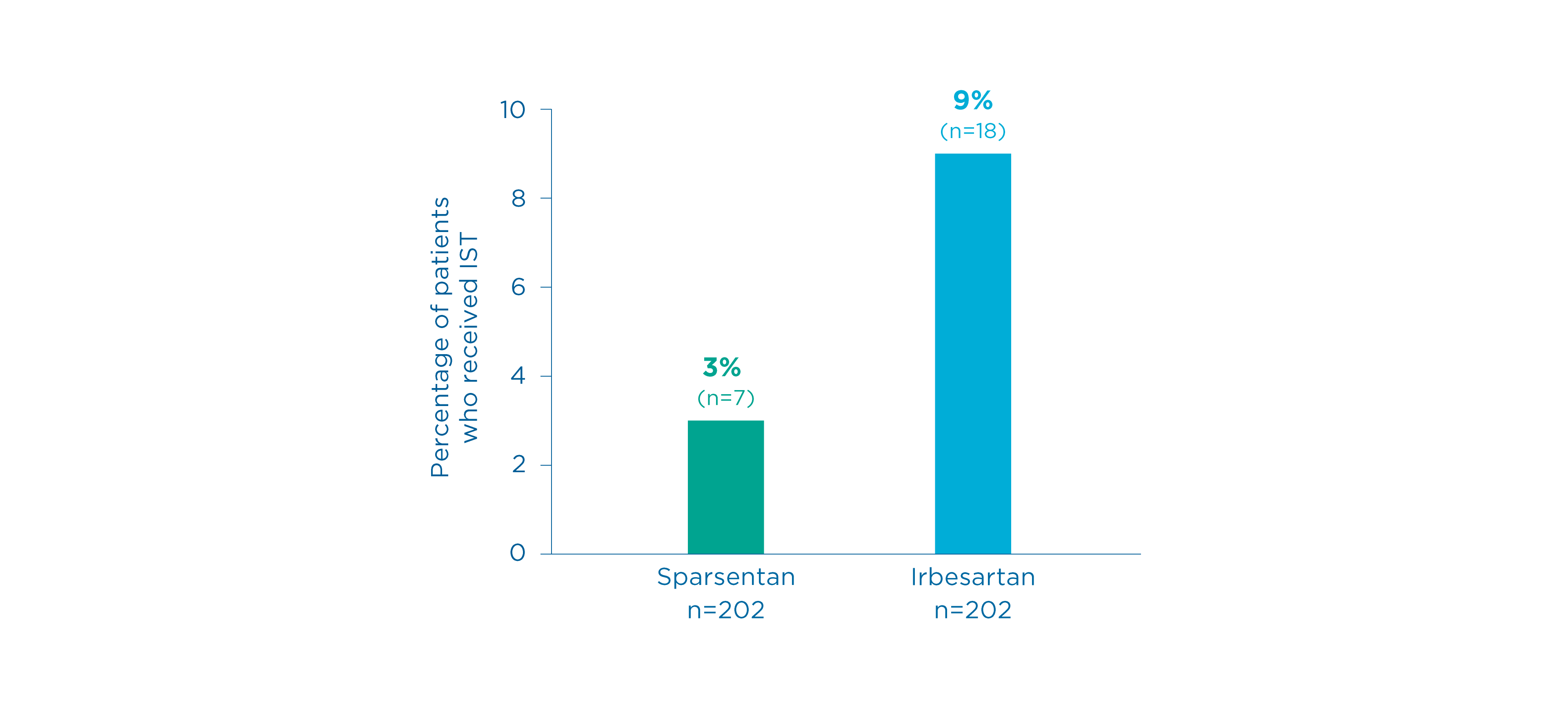
Use of IST in patients on sparsentan and irbesartan 5
Summary
- IST was initiated more frequently in the irbesartan group compared to the sparsentan group
5
- Sparsentan: 7 (3%) patients
- Irbesartan: 18 (9%) patients
-
Proteinuria (UPCR) at Week 36
Primary endpoint
-
Proteinuria (UPCR) at Week 110
Other prespecified secondary efficacy endpoint
-
Time to complete remission of proteinuria at Week 110
Prespecified exploratory endpoint
-
Complete and partial remission of proteinuria at Week 110
Prespecified exploratory endpoint
-
Estimated eGFR chronic slope or total slope at Week 110
Prespecified key secondary efficacy endpoint
-
Kidney function (eGFR) at Week 110
Other prespecified secondary efficacy endpoint
-
Composite kidney failure endpoint at Week 110
Other prespecified secondary efficacy endpoint
-
Potential long-term impact of improved eGFR slope
Additional analysis
-
Use of systemic IST through Week 110
Prespecified exploratory endpoint
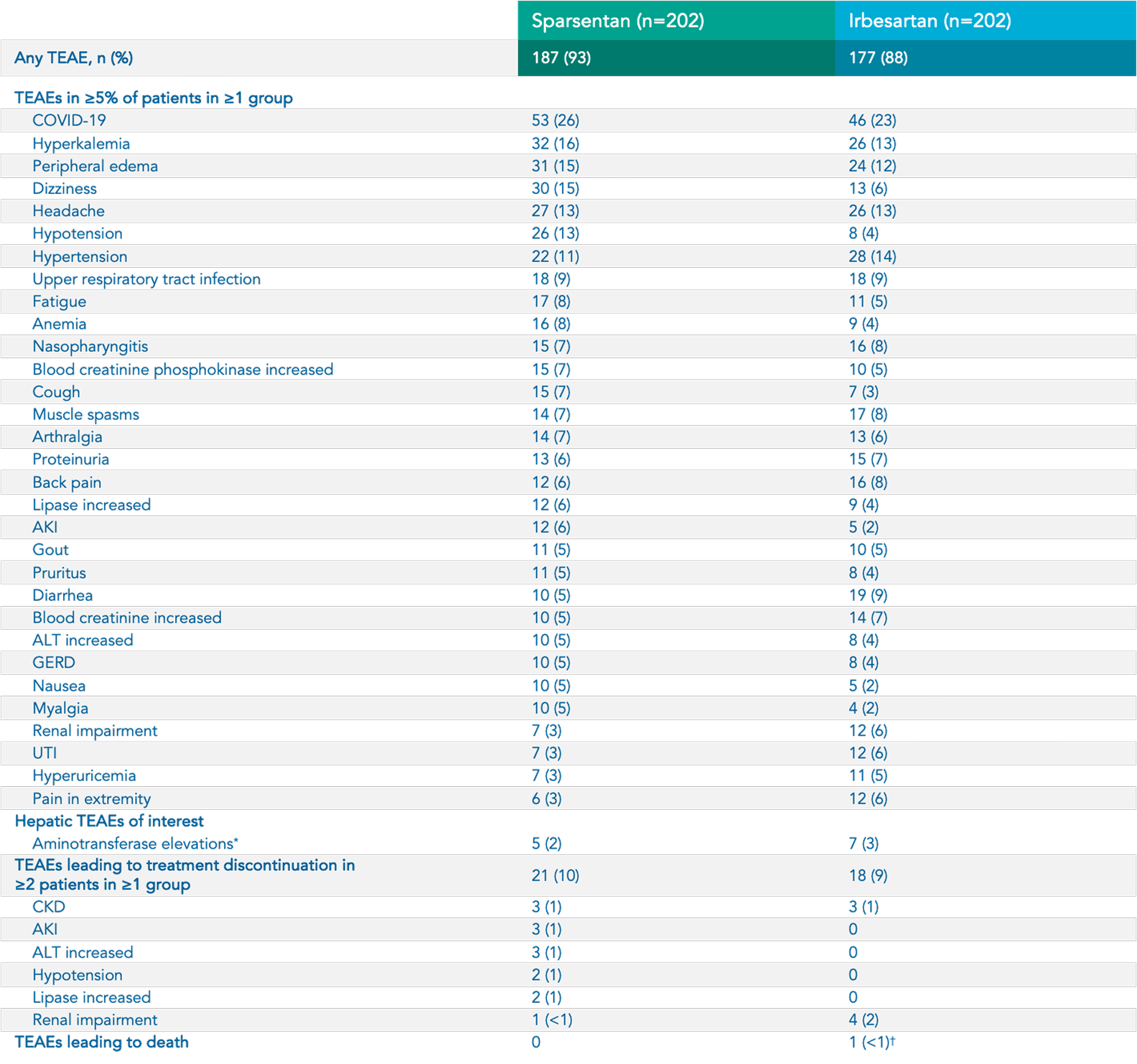
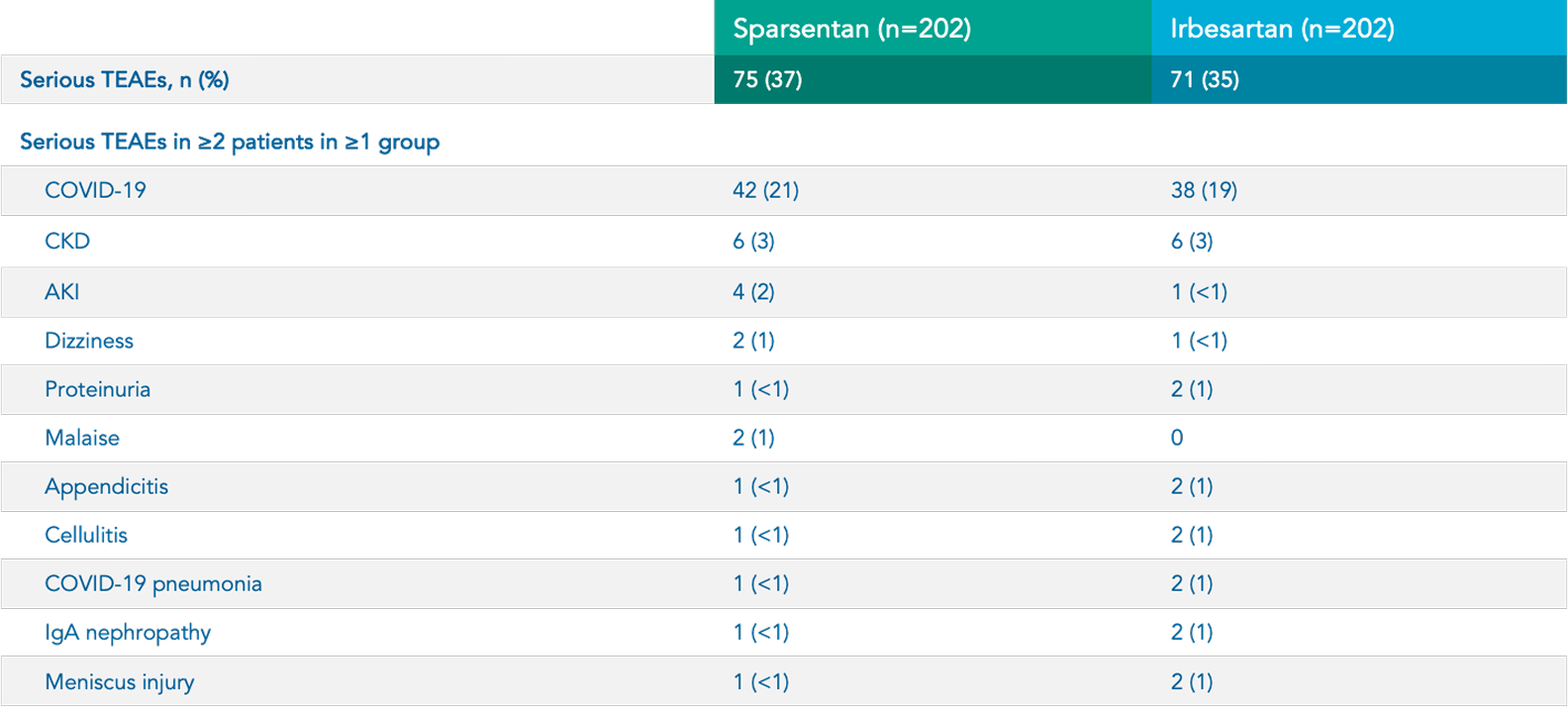
Safety
Sparsentan (n=202)
Irbesartan (n=202)

Hyperkalemia 5*
34 (17%)
27 (13%)

Hypotension 5
33 (16%)
13 (6%)

Peripheral
edema
5*
33 (16%)
29 (14%)

Dizziness 5*
32 (16%)
14 (7%)

Anemia 5
16 (8%)
9 (4%)

Acute kidney injury 5
12 (6%)
5 (2%)

Transaminase elevations 5†
7 (3.5%)
8 (4%)
In the sparsentan group, there were NO cases of 1 :

Discontinuations due to heart failure 1

Serious hepatic TEAEs or DILI 1

Serious TEAEs of drug-related edema or discontinuations due to edema 1
Footnotes:
*Includes related terms. 5
†Elevations in ALT or AST >3X ULN. 5
Sparsentan overview

Sparsentan is a novel, non-immunosuppressive,
Dual Endothelin Angiotensin Receptor Antagonist (DEARA)
14,
15