
In this issue we:
- Discuss the importance of proteinuria as a biomarker and the proposed lower proteinuria goals from the draft 2024 KDIGO (Kidney Disease: Improving Global Outcomes) guideline
- Highlight sparsentan data from ASN 2024 showing the impact of lower proteinuria on kidney preservation in multiple IgA nephropathy populations
Proteinuria is the single strongest modifiable prognostic indicator for measuring progression of IgA nephropathy1,2
To prolong kidney survival and address the clinical unmet needs of IgA nephropathy, the evolving care paradigm emphasizes lowering proteinuria goals.3,4
Proteinuria is established as the only validated biomarker for measuring IgA nephropathy progression.1-3 We now know that patients traditionally considered low risk for disease progression (i.e. proteinuria levels <1.0 g/day) treated with renin-angiotensin system inhibitors (RASi) often progress to kidney failure within 10 years as demonstrated by data from the IgA nephropathy cohort in the RaDaR study.4
The newly released draft 2024 KDIGO guideline recommends targeting proteinuria levels <0.5 g/day, preferably <0.3 g/day (complete remission [CR]) to prolong kidney survival.3
ASN 2024 data suggest that sparsentan can achieve 2024 draft KDIGO guideline proteinuria treatment goals in multiple IgA nephropathy patient populations1,5-7
Safety and efficacy of sparsentan in patients with IgA nephropathy was established in PROTECT, an active-controlled Phase 3 study.8 Patients treated with sparsentan in the PROTECT study were nearly three times more likely to achieve CR (<0.3 g/day) as compared to those treated with maximum labeled dose irbesartan.8
At ASN 2024, additional PROTECT analyses demonstrated the importance of lowering proteinuria and the clinical implications of using sparsentan to reach proteinuria remission in various IgA nephropathy patient populations.5,6
In a PROTECT post hoc analysis of proteinuria remission, the kidney function of patients achieving or not achieving CR were assessed.5 Regardless of whether randomized to active control (i.e. irbesartan) or sparsentan, patients who achieved urinary protein excretion (UPE) <0.5 g/day showed a slower estimated glomerular filtration rate (eGFR) decline indicating that reaching low proteinuria target levels was predictive of better long-term kidney function.5
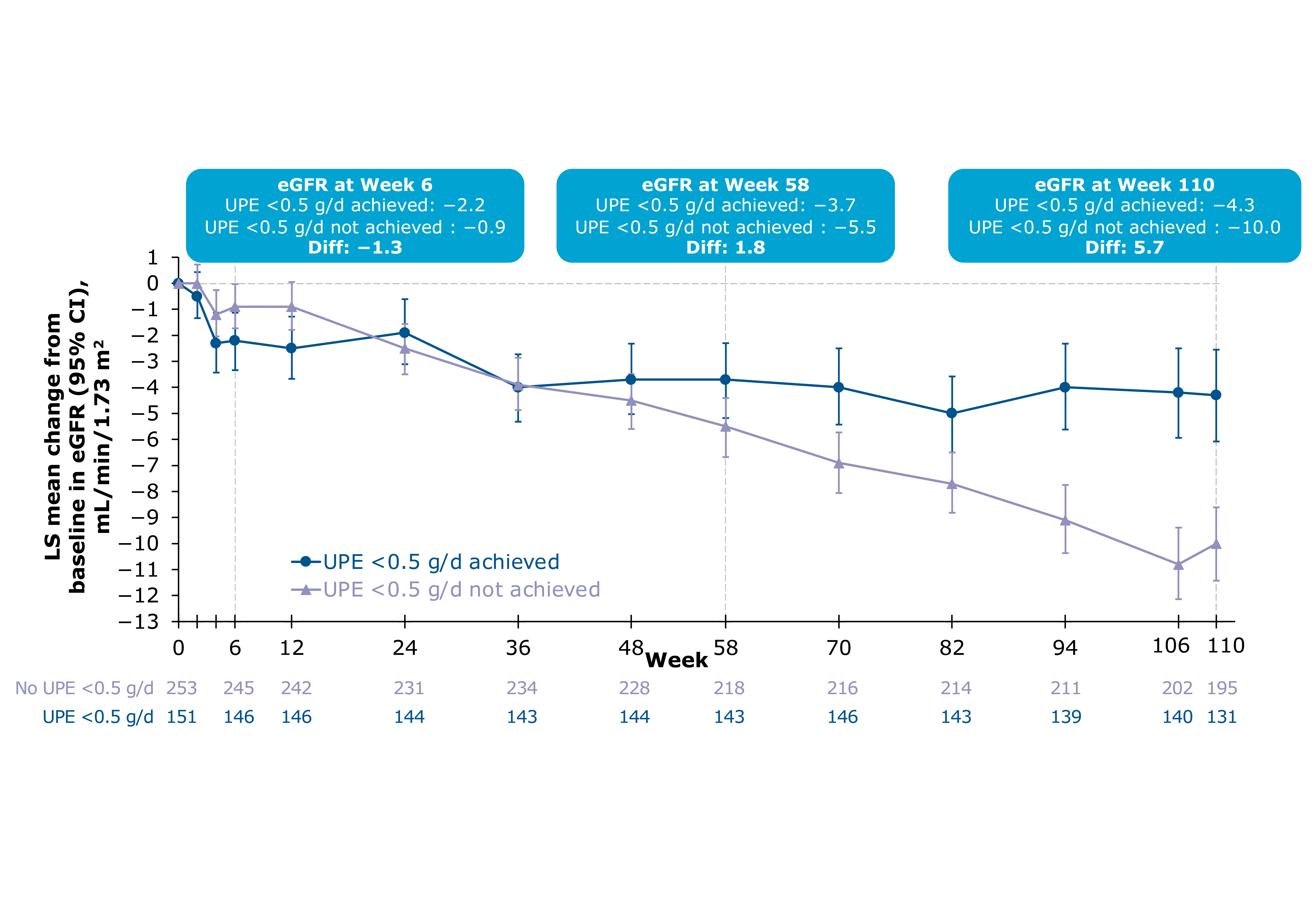
Figure. PROTECT post hoc analysis: Absolute change from baseline in eGFR at each study visit in patients who did or did not achieve UPE <0.5 g/day5a
aChange from baseline in eGFR by visit using a mixed models for repeated measures (MMRM) with on-study values and baseline UPE adjustment.5
In PROTECT, 37% (151/404) of patients, irrespective of treatment assignment, achieved UPE <0.5 g/day at any time during the study.5 Of those who achieved UPE<0.5 g/day, 68% (103/151) of patients were treated with sparsentan, and 32% (48/151) were treated with maximum labeled dose irbesartan.5
Explore more data from the PROTECT post hoc analysis of proteinuria remission.
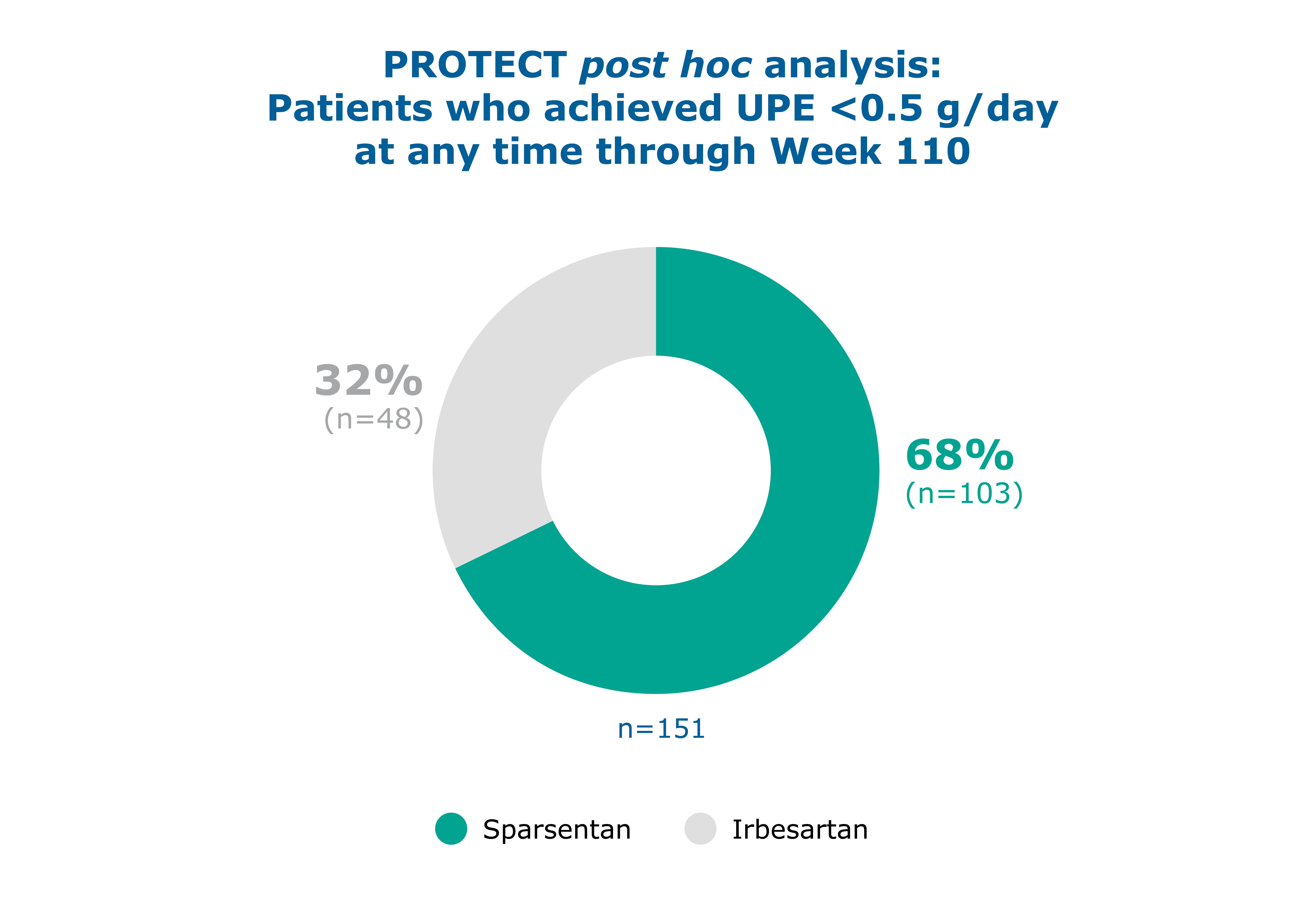
Figure. PROTECT post hoc analysis: Patients who achieved UPE <0.5 g/day at any time through Week 1105
In a separate post hoc subgroup analysis, the probability of achieving CR at any time during the study by baseline urine protein-creatinine ratio (UPCR) values above and below 1.0 g/g was studied.6
In this analysis, CR was achieved earlier and more frequently in patients treated with sparsentan versus irbesartan, regardless of baseline UPCR level.6
Explore more data from this PROTECT post hoc subgroup analysis.
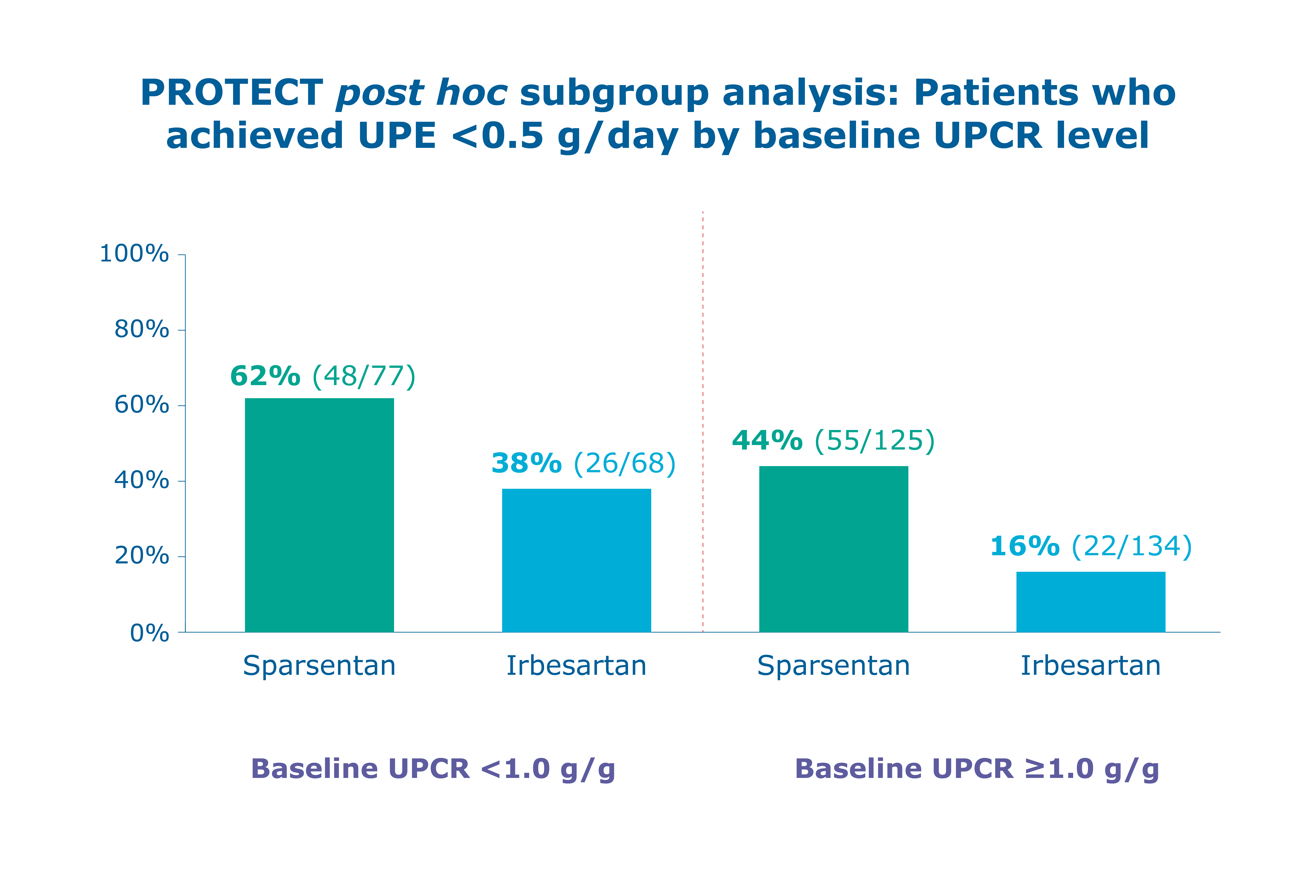
Figure. PROTECT post hoc subgroup analysis: Patients who achieved UPE <0.5 g/day by baseline UPCR level6
In PROTECT and its post hoc analyses, patients receiving sparsentan had greater proteinuria reduction and achieved proteinuria remission earlier and more frequently than patients on maximum labeled dose irbesartan, and sparsentan slowed the rate of eGFR decline to a greater degree regardless of baseline UPCR.5,6,8
Sparsentan was well tolerated with a comparable safety profile to maximum labeled dose irbesartan.5,6
While PROTECT showed the importance of complete proteinuria remission in previously treated patients with IgA nephropathy, the interim analysis of SPARTAN, a Phase 2, open-label, single arm, multicenter trial, demonstrated the effect of sparsentan in treatment-naive patients.5-7
Newly diagnosed patients being treated with sparsentan experienced rapid and sustained reduction in proteinuria (approximately 70% from baseline).7 Of those patients, 75% (9/12) achieved UPE <0.5 g/day at any time point in the 24-week SPARTAN study.7
No new safety signals were identified in this interim analysis of SPARTAN data.7
Explore more data from the SPARTAN interim analysis.
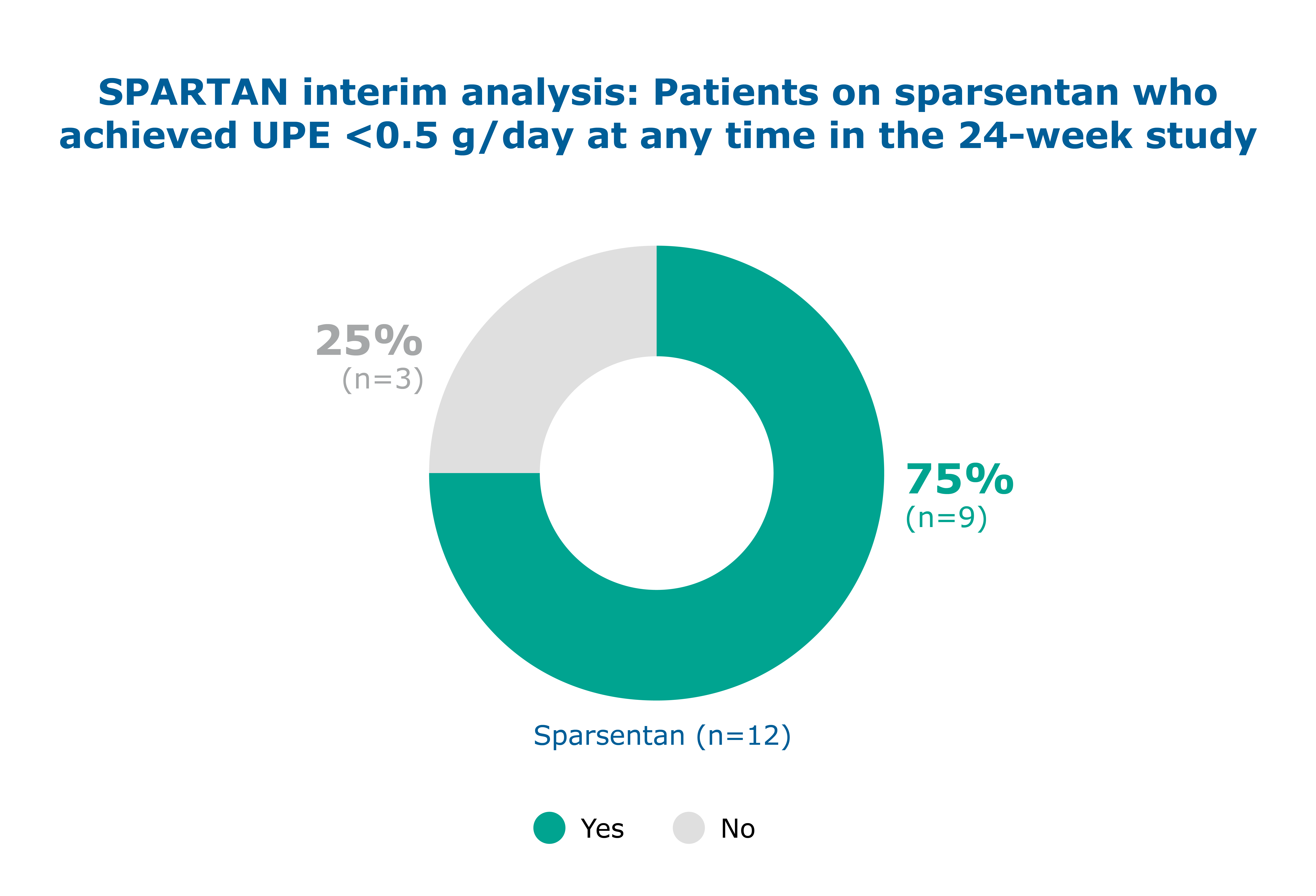
Figure. SPARTAN interim analysis: Patients who achieved UPE <0.5 g/day at any point in the 24-week study7
In conclusion, data from the PROTECT and SPARTAN analyses demonstrated that patients on sparsentan can reach the proteinuria treatment goals outlined in the 2024 KDIGO draft guideline, benefitting multiple patient populations including1,5-7:
- Previously treated patients
- Patients once considered low risk (UPCR <1.0 g/day)
- Treatment-naïve patients
ASN Kidney Week 2024
Learn more about Travere Therapeutics’ data from ASN 2024
Footnotes
CR, complete remission; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes; MMRM, mixed models for repeated measures; RASi, renin-angiotensin system inhibitor; UPE, urinary protein excretion; UPCR, urine protein-creatinine ratio.
- Inker LA et al. Am J Kidney Dis. 2016;68(3):392-401.
- Reich HN et al. J Am Soc Nephrol. 2007;18(12):3177-3183.
- KDIGO Clinical Practice Guideline for the Management of Immunoglobulin A Nephropathy (IgAN) and Immunoglobulin A Vasculitis (IgAV). Accessed 22 October 2024. https://kdigo.org/wp-content/uploads/2024/08/KDIGO-2024-IgAN-IgAV-Guideline-Public-Review-Draft.pdf
- Pitcher D et al. Clin J Am Soc Nephrol. 2023;18(6):727-738.
- Heerspink JLH et al. Poster presented at: American Society of Nephrology 2024; October 23-27, 2024; San Diego, USA. FR-PO872.
- Kooienga I et al. Poster presented at: American Society of Nephrology 2024; October 23-27, 2024; San Diego, USA. FR-OR57.
- Cheung CK et al. Presented at: American Society of Nephrology 2024; October 23-27, 2024; San Diego, USA. FR-OR63.
- Rovin BH et al. Lancet. 2023;402(10417):2077-2090.
MA-SP-24-0176 | December 2024