Sparsentan. Dual Angiotensin II AT1 Receptor Blocker and Endothelin ETA Receptor Antagonist, Treatment of Focal Segmental Glomerulosclerosis, Treatment of IgA Nephropathy
Drugs of the Future – 2020
Endothelin-1 (ET-1) and angiotensin II (Ang II)
ET-1 and Ang II are important disease mediators in chronic progressive kidney disease such as IgA nephropathy and focal segmental glomerulosclerosis (FSGS)1
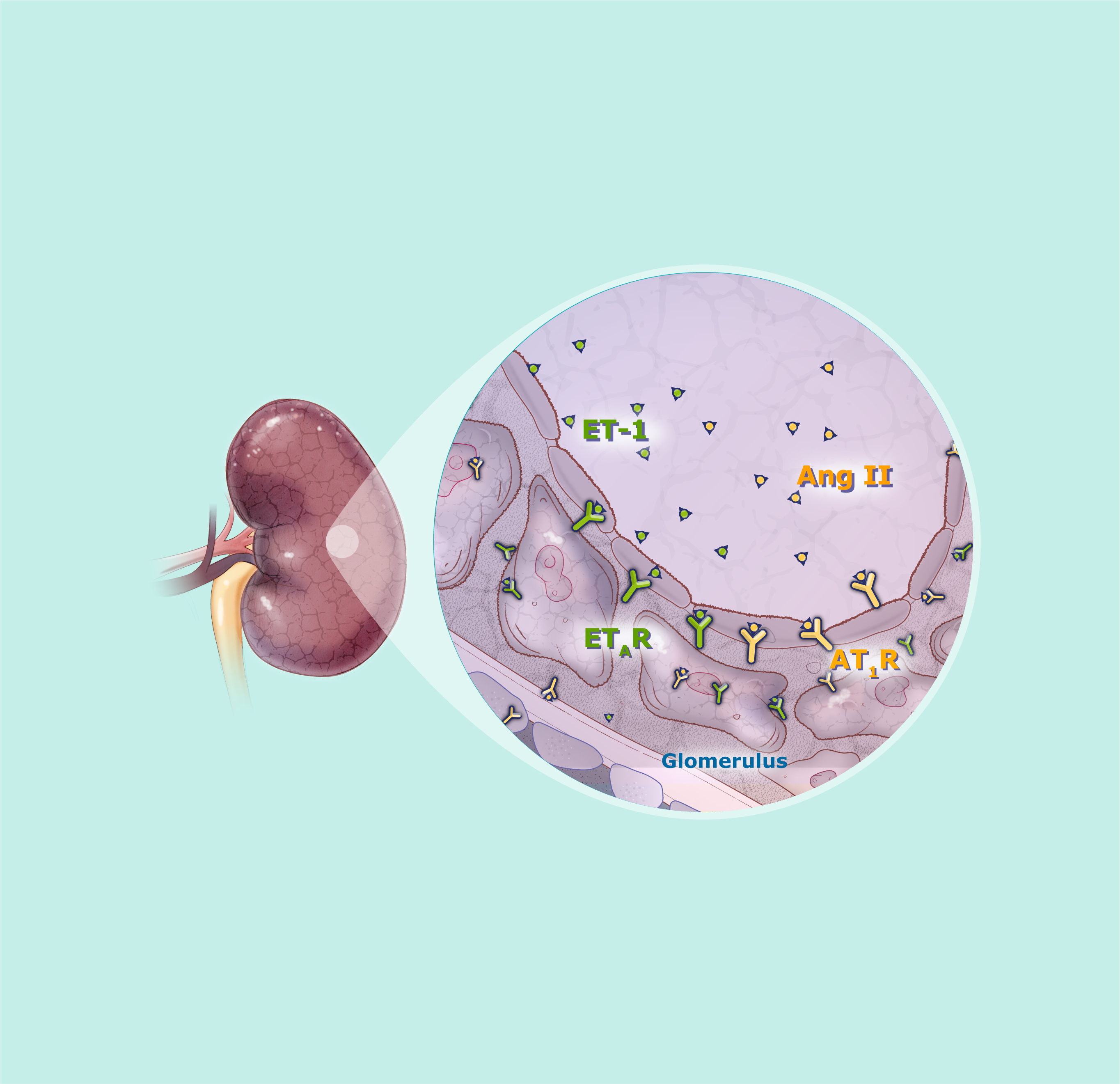
ET-1 and Ang II are present throughout the kidney
Class and structure of sparsentan
Sparsentan is a Dual Endothelin Angiotensin Receptor Antagonist (DEARA), with nephroprotective effects1
The sparsentan molecule consists of1:
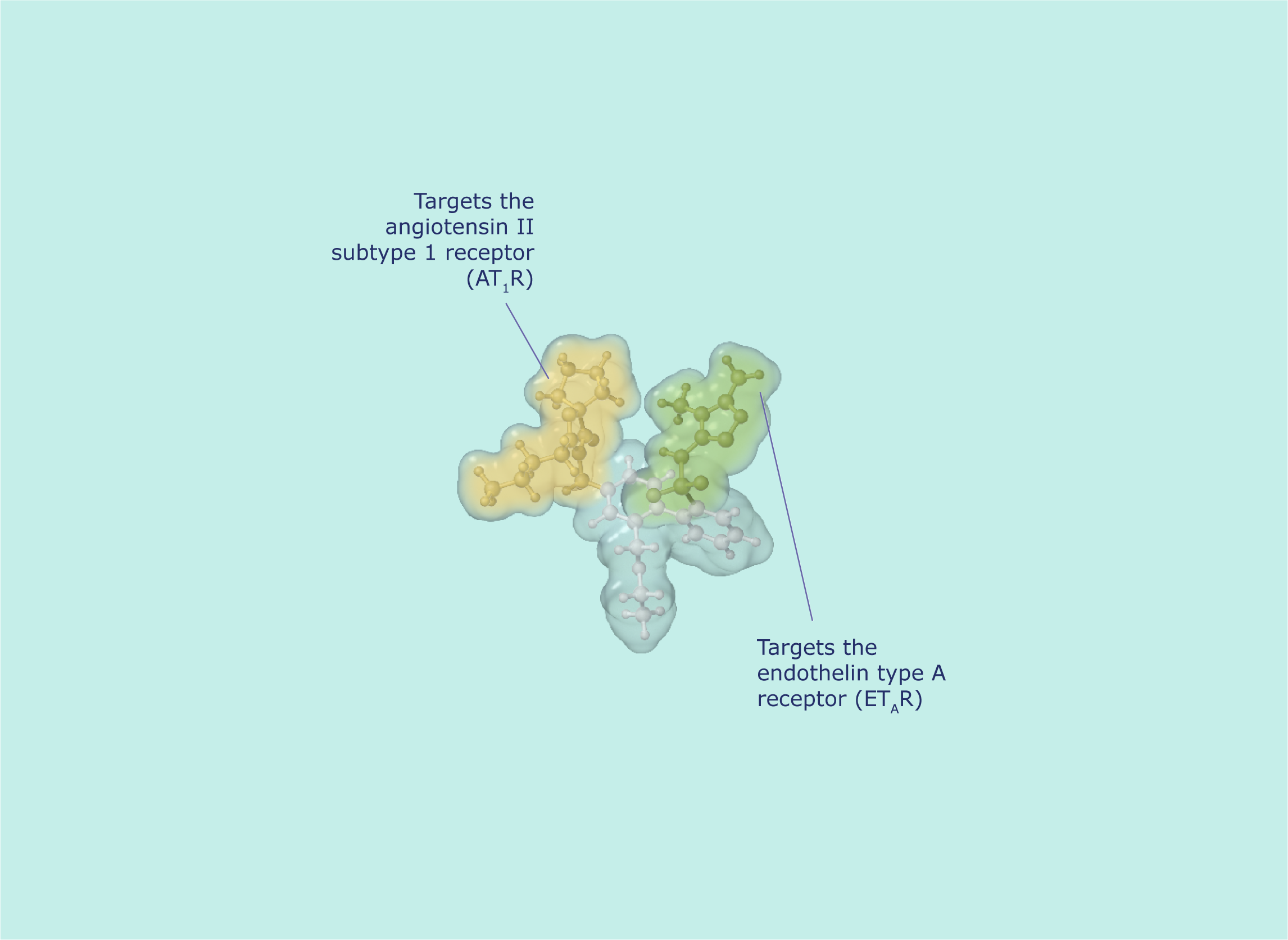
The molecular structure of sparsentan targets ETAR and AT1R
Sparsentan targets glomerular injury and slows kidney function decline through several protective effects1,7
Preclinical data support the direct cellular, and resulting structural, actions of sparsentan1*
| In IgA nephropathy | In FSGS |
|---|---|
| Anti-inflammatory8-11* | Podocyte-protective13,14* |
| Anti-proliferative8,9,11* | Glycocalyx-protective14* |
| Anti-fibrotic10,11* | Glomerular vasodilation13,14* |
| Anti-proteinuric12 | Anti-fibrotic13* |
| Anti-inflammatory15* | |
| Anti-proteinuric16,17 |
*These effects are based on pre-clinical animal modeling data.
Resources
Conclusions
Collectively, the protective effects of sparsentan contribute to nephroprotection in kidney disease1
As of October 2024, sparsentan is not FDA approved for the treatment of FSGS.
Ang II, angiotensin II; AT1R, angiotensin II subtype 1 receptor; DEARA, Dual Endothelin Angiotensin Receptor Antagonist; ET-1, endothelin-1; ETAR, endothelin type A receptor; FSGS, focal segmental glomerulosclerosis; IgA, immunoglobulin A.
MA-SP-24-0108 | April 2025