In the Phase 2 SPARTAN open-label, single-arm study (N=12), treatment with sparsentan demonstrated proteinuria reduction independent of changes in blood pressure and a favorable safety profile consistent with the Phase 3 PROTECT study in a treatment-naïve population1,2
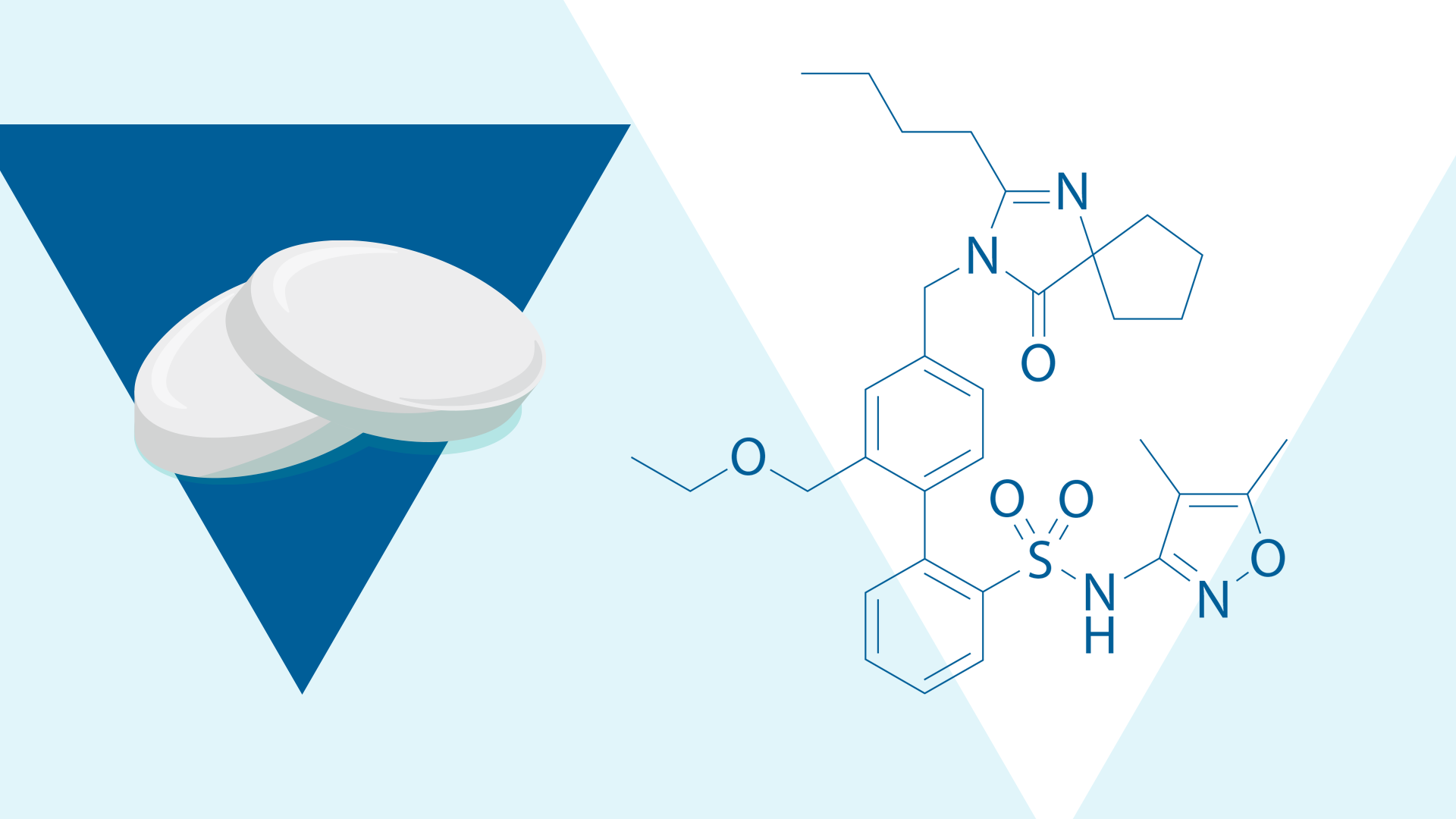
Sparsentan is a non-immunosuppressive Dual Endothelin Angiotensin Receptor Antagonist (DEARA) approved in the US to slow kidney function decline in adults with IgA nephropathy who are at risk for disease progression.2-5 In preclinical models of IgA nephropathy, sparsentan has demonstrated anti-inflammatory, anti-fibrotic, anti-proliferative,* and anti-proteinuric effects, suggesting a multi-pathway impact on kidney injury.3,6-9
SPARTAN examined the effects of sparsentan on the underlying pathophysiology of IgA nephropathy, incorporating a biomarker-focused approach.1,2 Over a 24-week treatment period, the study evaluated proteinuria reduction and a broad panel of urinary biomarkers reflecting inflammation, fibrosis, and kidney injury.1,2

Figure. SPARTAN study design1
In the SPARTAN study, treatment with sparsentan led to rapid and sustained reductions in proteinuria over the 24-week study period.2 Patients with IgA nephropathy experienced a ~70% decrease in urine protein-creatinine ratio (UPCR) from baseline.2 Median UPCR declined from 1.3 g/g (IQR: 0.4-1.7) at baseline to 0.3 g/g (IQR: 0.1-0.6) at Week 24.2

Figure. Geometric mean change from baseline in UPCR1
Estimated glomerular filtration rate (eGFR) levels remained relatively stable throughout the 24-week study period.1 Read more about eGFR results in the SPARTAN study
Similarly, systolic and diastolic blood pressure (SDP and DBP) remained stable throughout the 24-week treatment period, with only a slight initial decrease observed following the start of treatment.1

Figure. Mean blood pressure at each visit over 24 weeks1
Overall, sparsentan was generally well tolerated over 24 weeks of treatment.1,2 One patient permanently discontinued treatment due to hypotension after Week 6.1

Figure. Patients with any AE1
Consistent with preclinical findings, sparsentan showed improvement in inflammatory and profibrotic biomarkers2
In preclinical models of IgA nephropathy, including the gddY mouse, the IgA1-IgG engineered immune complex (EIC) mouse, and the anti-Thy1 rat, sparsentan reduced expression of immune, inflammatory, and fibrotic gene pathways in whole-kidney tissue.7,9

Targeting Inflammation and Fibrosis in IgA Nephropathy: Preclinical Data and Translational Biomarker Insights
Revisit part one for a refresher on inflammation and fibrosis in IgAN, preclinical sparsentan data, and biomarker rationale.
In SPARTAN, a wide panel of urinary biomarkers was used to evaluate the impact of sparsentan on inflammation and fibrosis in IgA nephropathy2
Notably, decreases were observed in soluble CD163 (sCD 163) and interleukin 6 (IL-6), with sCD163 acting as a marker of M2c macrophage activation and IL-6 acting as a marker of inflammatory signaling.2,10

Figure. Changes in urinary biomarkers at each visit over 24 weeks2
Sparsentan treatment was also associated with reduced urinary B-cell activating factor (BAFF) and soluble C5b9 (sC5b9), suggesting downregulation of B-cell and complement activation pathways.2

Figure. Change in BAFF and sC5b9 at each visit over 24 weeks2

Urinary Biomarker Analysis Reveals Rapid Intrarenal Anti inflammatory and Anti-fibrotic Effects of Sparsentan in IgA Nephropathy in the SPARTAN Study
Learn more about interim SPARTAN data on urinary biomarkers reflecting intrarenal inflammation, fibrosis, and injury in IgA nephropathy.
Clinical and biomarker findings from the SPARTAN study show that treatment with sparsentan was well tolerated and led to rapid and sustained reductions in proteinuria and urinary biomarkers of inflammation and fibrosis.1,2
Data from this study contribute to the mechanistic rationale for sparsentan and provide a framework for future validation of biomarker-guided approaches in larger studies, such as PROTECT.1,2 Furthermore, integrating biomarker data with clinical outcomes may help further understanding of the underlying mechanisms contributing to sparsentan clinical efficacy.1,2
Together, these interim data support the role of sparsentan as a kidney-targeted therapy and reinforce the importance of developing urinary biomarkers to help to monitor disease progression and treatment response in IgA nephropathy.2

Insights from the SPARTAN Study with Drs. Jonathan Barratt and Shikha Wadhwani
Hear experts discuss how SPARTAN biomarker data may offer insights into kidney-localized inflammation and fibrosis in IgA nephropathy.
Related Content
Targeting Inflammation and Fibrosis in IgA Nephropathy: Preclinical Data and Translational Biomarker Insights
Explore inflammation and fibrosis in IgAN , preclinical sparsentan findings, and key urinary biomarkers
Exploring the Mechanism of Sparsentan in IgAN: Including a Spotlight on Urinary sCD163, a Key Marker of Inflammation
Learn more about the role of u-sCD163 in IgAN and the biomarker findings from the SPARTAN interim analysis!
*These effects are based on pre-clinical animal modeling data.7
†One patient experienced a serious AE, which was a limb abscess.1
‡α2M, clusterin, and plasminogen analysis was performed only at baseline and Week 12.1
References
- Cheung CK et al. Presented at: American Society of Nephrology, 2024; October 23-27, 2024; San Diego, USA. FR OR63.
- Cheung CK et al. Presented at: International Podocyte Conference & ISGD Meeting, 2025; June 10-13, 2025; Hamburg, Germany. FR_11.
- Kohan DE et al. Clin Sci (Lond). 2024;138(11):645-662.
- Rovin BH et al. Lancet. 2023;402(10417):2077-2090.
- FILSPARI® (sparsentan) Prescribing Information. San Diego, CA: Travere Therapeutics, Inc. 8/2025.
- Jenkinson C et al. Poster presented at: ISN World Congress of Nephrology, 2019; April 12-15, 2019; Melbourne, Australia. SAT-010.
- Reily C et al. Am J Physiol Renal Physiol. 2024;326(5):F862-F875.
- Nagasawa H et al. Nephrol Dial Transplant. 2024;39(9):1494 1503.
- Jenkinson C et al. Presentation at: International Symposium on IgA Nephropathy, 2018; September 27-29, 2018; Buenos Aires, Argentina.
- Li J et al. Kidney International Reports. 2024;9(10):3016-3026.
MA-SP-25-0133 | November 2025