
Rare Kidney Disease Show Podcast
Learn more about the endothelin system and how it relates to IgAN pathophysiology
Listen now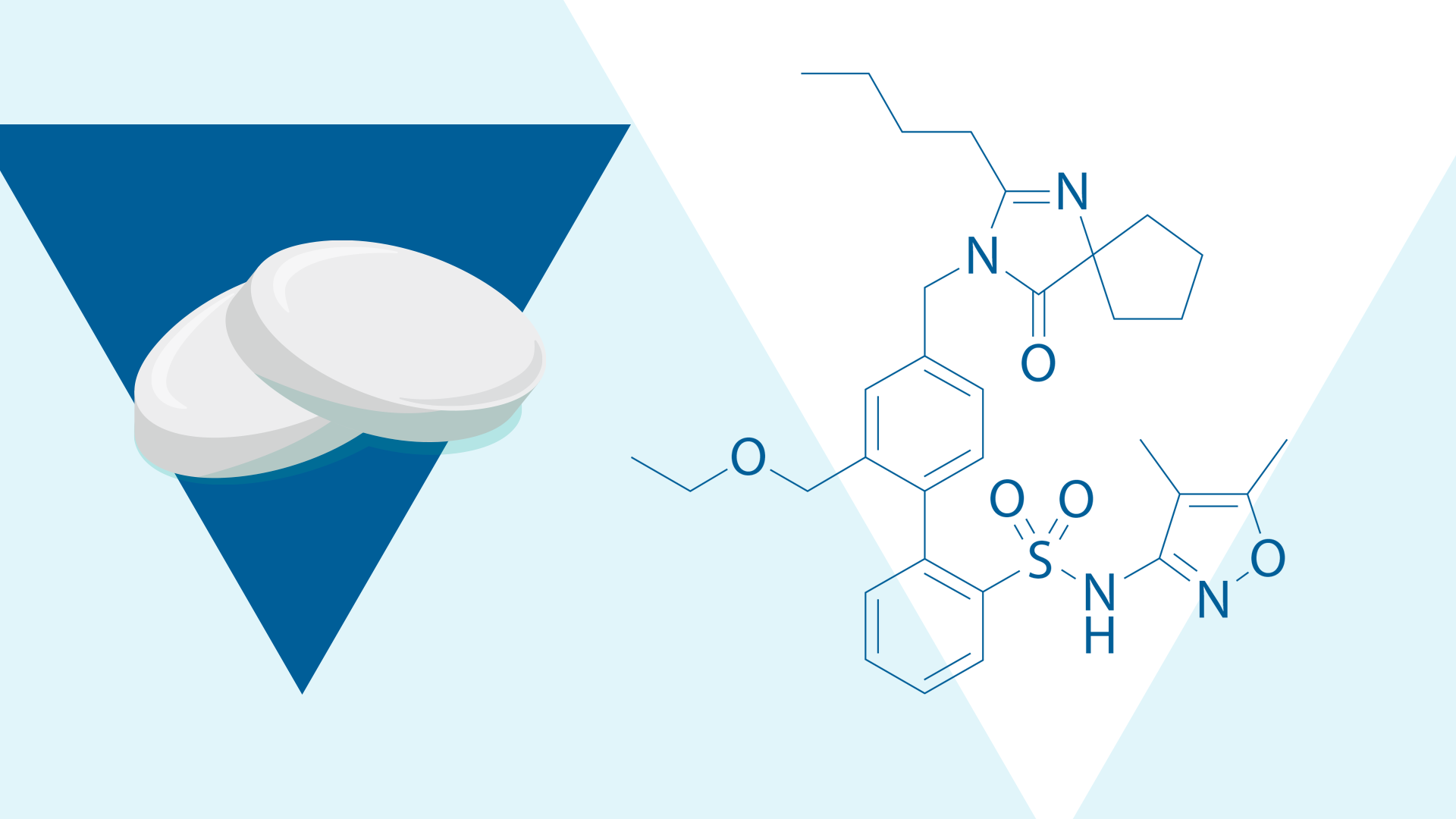
In this issue we:
Ang II and ET-1 are key disease mediators that contribute to the progression of IgA nephropathy.1 They upregulate each other and exert an additive effect driving ongoing kidney damage.1-3
Ang II impacts nearly the entire kidney through activation of angiotensin II subtype 1 receptors (AT1R), and ET-1 activity in the kidney is mostly mediated through the endothelin type A receptor (ETAR).1
Both Ang II and ET-1 have similar pathophysiological functions within the kidney that contribute to actions such as vasoconstriction, inflammation, and cellular proliferation.1,4,5 Collectively, these effects contribute to hypertension, glomerulosclerosis, proteinuria, and tubulointerstitial fibrosis.1,4,6
Preclinical data suggest that increased ET-1 leads to inflammation, particularly in the glomerulus.7-10

Rare Kidney Disease Show Podcast
Learn more about the endothelin system and how it relates to IgAN pathophysiology
Listen nowWithin the glomerulus, ET-1 is constitutively expressed in all cells and is involved in the homeostasis of glomerular structure and filtration function.7 Findings indicate that ET-1 increases glomerular permeability to proteins and renal inflammation.7-10
• Mesangial cells: Increased ET-1 causes cellular proliferation, contraction, and increased extracellular matrix accumulation.7,10 Excessive ET-1 activity in mesangial cells may be strongly indicative of glomerular damage.10
• Endothelial cells and podocytes: Increased ET-1 production leads to glomerular inflammation and injury, sclerosis, and podocyte injury, all contributing to proteinuria.7
• Glycocalyx: The IgA nephropathy-prone gddY mouse model demonstrated an upregulation of ET-1 and ETAR and a decrease in the glycocalyx area.8
Ultimately blocking ETAR could mediate glomerular damage and the proinflammatory effects of ET-1.7-9

Figure. Renal ET-1 and ETAR increases in IgA nephropathy11
Several biomarkers have been associated with inflammation in IgA nephropathy including urinary soluble CD163 (u-sCD163).12,15,16
CD163 is a transmembrane protein expressed during the healing phase of inflammation.12 It is a marker of alternatively activated macrophages and inflammation in several proinflammatory diseases.13,17,18
In the kidney, CD163 has been correlated with macrophage infiltration and is associated with active lesions.15
In IgA nephropathy, u-sCD163 has been associated with inflammation in the TESTING and NEFIGAN studies.15,16
The recent Phase 2 SPARTAN interim analysis, presented at ASN Kidney Week 2024 and WCN 2025, assessed u-sCD163 as an inflammation biomarker in patients with IgA nephropathy treated with sparsentan.19
Early mouse models of IgA nephropathy found that sparsentan decreased glomerular macrophage infiltration and attenuated immune-complex mediated mesangial cellularity.21,22 Recent IgA nephropathy mouse models have demonstrated that treatment with sparsentan can attenuate gene expression specific to immune and inflammatory pathways, including inflammatory pathway gene markers.8,20

Figure. Sparsentan decreased expression of immune and pro-inflammatory signaling in kidneys of IgA nephropathy-prone gddY mice8
Interim u-sCD163 data from SPARTAN (n=11), a Phase 2, open-label, single-arm multicenter trial, demonstrated the anti-inflammatory effect of sparsentan.19
Findings showed a rapid and sustained reduction of u-sCD163 with sparsentan treatment.19 At Week 24, there was a 50% reduction in u-sCD163 from baseline indicating a reduction in inflammation in patients.19

Figure. u-sCD163 levels decreased over 24 weeks with sparsentan19
The SPARTAN study will continue to evaluate additional biomarkers to better elucidate the additional mechanistic actions of sparsentan, a novel, non-immunosuppressive Dual Endothelin Angiotensin Receptor Antagonist (DEARA).1,19,23
Over the last two decades, preclinical data has implicated ET-1 in renal inflammation.7,9,10 Recently, biomarker data from the SPARTAN interim analysis demonstrated sparsentan’s anti-inflammatory effect in humans, supporting data from early mouse models of IgA nephropathy that showed attenuation of immune and proinflammatory signaling with sparsentan.8,19,20

A New Dawn for IgA Nephropathy – Redefining What is Possible with Sparsentan
Travere and ISN partnered to host a webinar on the place of sparsentan in the evolving treatment landscape of IgA nephropathy.
Explore the webinar details now.
MA-SP-25-0016 | April 2025